Smart Ways to Find the Limiting Reactant in 2025
Understanding the Limiting Reactant
In any chemical reaction, key concepts lie in the balancing of reactants and products. The limiting reactant is critical because it determines the maximum amount of product that can be formed. By recognizing which reactant runs out first, chemists can accurately calculate yields, making it fundamental in reactant calculations. This article explores how to effectively find the limiting reactant using various techniques, emphasizing the importance of stoichiometry in achieving accurate results in chemical reactions.
What is a Limiting Reactant?
The limiting reactant is defined as the substance that is entirely consumed during a chemical reaction, hence limiting the extent of the reaction. Understanding the concept begins with recognizing the stoichiometric coefficients in a balanced equation. These coefficients inform the mole ratio of reactants that we need to test against the actual amounts available in a reaction mixture. For instance, in a reaction where hydrogen and oxygen combine to form water, if there are twice as many hydrogen molecules as oxygen, oxygen is the limiting reagent because all its molecules will be used before the hydrogen runs out.
Step-by-Step Method for Finding the Limiting Reactant
To accurately determine the limiting reactant, follow these steps:
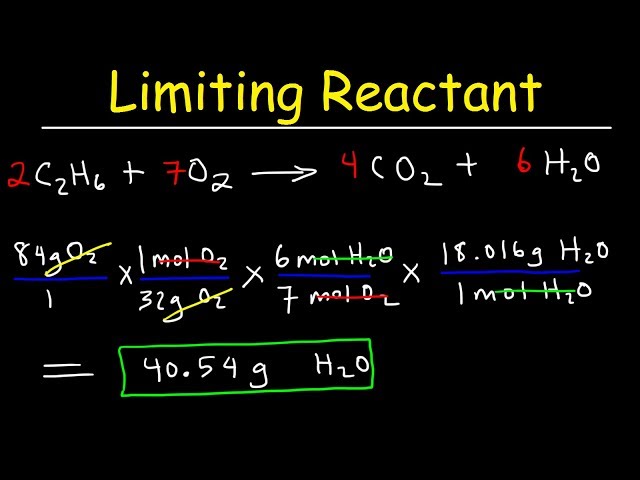
- Write the Balanced Equation: Draft the chemical equation for the reaction, ensuring that it is balanced. For example, for the formation of water (2H₂ + O₂ → 2H₂O), we see that two moles of hydrogen react with one mole of oxygen.
- Identify Mole Ratios: Use the coefficients from the balanced equation to ascertain the necessary mole ratios required for the reaction.
- Convert to Moles: Convert the masses of your reactants into moles using their respective molar masses. This is essential for accurate calculations.
- Calculate Reactant Amounts: Using the mole ratios, compare how much of each reactant is needed versus how much is available.
- Identify the Limiting Reactant: The reactant that runs out first is your limiting reagent, determining how much product can be formed.
Real-World Application of Limiting Reactants
The concept of the limiting reactant plays a vital role in various industries, like pharmaceuticals and manufacturing. For instance, in drug synthesis, determining the limiting reactant not only ensures optimal use of materials but also minimizes waste, enhancing reaction efficiency. Practice in calculating limiting reactants is essential for students and professionals alike, as accurate yield calculations lead to cost-effective processes. Moreover, incorporating these principles into laboratory experiments fosters a practical understanding of stoichiometry that is valuable across various chemical reaction contexts.
Techniques for Efficient Reactant Comparison
To enhance your ability to efficiently find the limiting reactant, several techniques can be applied that utilize both qualitative and quantitative analysis.
Using Graphical Representations
Visualizing chemical reactions using graphical representations such as charts or graphs can help identify the limiting reactant more quickly. By plotting reactant amounts against time, you can often see which reactant declines first, providing an immediate understanding of which is limiting. For example, during an acid-base reaction, you can graph the pH level over time to gauge when the reactants have fully reacted, indicating the establishment of limiting and excess reactants.
Employing Stoichiometric Calculators
Various tools and calculators available online can simplify the process of limiting reactant calculations. By entering the relevant mole ratios and reactant amounts, these tools quickly determine which reactant limits the reaction. Educational resources alongside such tools enhance comprehension and application of stoichiometry in cooking, illustrating how these principles extend into everyday situations.
Experimental Techniques for Classrooms
Engaging students in practical lessons where they apply stoichiometric calculations helps solidify concepts of limiting reactants. For example, performing controlled reactions in laboratory settings enhances learning. After conducting the reaction, students can measure product quantity and compare it to theoretical yields to better understand wastage, error margins, and the efficiency of reactants. Students can also observe common laboratory techniques and learn the significance of precise measurements. This practice will lead to better laboratory skills over time.
Troubleshooting and Common Misconceptions
Many students struggle with the concept of limiting reactants, leading to several misconceptions. A clear understanding of basic chemistry concepts can rectify these misunderstandings and contribute positively to student outcomes in chemistry education.
Identifying Missteps in Calculations
A common misstep in determining the limiting reactant occurs when students overlook the importance of fully balancing their chemical equations. Without a full and accurate balance, the stoichiometric relationships that are critical for proper calculations will be incorrect. Hence, adherence to careful chemical equation balancing cannot be overstated in this context, as it ensures that all reactant interactions are appropriately accounted for.
Clarifying the Function of Excess Reactants
Understanding the role of the excess reactant is equally important. Students often misinterpret the excess quantity as being irrelevant; however, knowing the excess amount can assist in clarifying how the limiting reactant impacts the entire reaction pathway. For example, correctly calculating the leftover excess reactant provides insights into reaction completeness and optimization potential in industrial applications.
Utilizing Significant Figures
In chemistries, such as reactant calculations, using proper significant figures is vital. Overlooking this detail can lead to inaccuracies in determining yields and understanding experimental outcomes. When conducting calculations, students should always align their final results with the precision of the measurements taken during the experiment. Upholding this standard improves accuracy and fosters better practices in scientific inquiry through practical experiments.
Key Takeaways
- The limiting reactant determines the amount of product formed in a chemical reaction.
- A balanced equation is crucial for identifying the correct mole ratios.
- Graphical visualizations and stoichiometric calculators can greatly simplify reactant comparison.
- Hands-on experiments solidify the practical understanding of stoichiometry.
- Awareness of significant figures and common missteps in calculations reinforces high standards in chemical practice.
FAQ
1. How do I determine the limiting reactant in a complex reaction?
To pinpoint the limiting reactant in complex reactions, begin with a detailed chemical equation, balance it correctly, and utilize **stoichiometric coefficients**. Then calculate the moles of the available reactants and see which one runs out first based on the established mole ratios.
2. Why is finding the excess reactant important?
Identifying the **excess reactant** helps in understanding how complete the reaction was and can indicate areas for improving efficiency in future experiments or industrial processes. It also assists in estimating the cost-effectiveness of reagents used.
3. Can limiting reactants vary in different conditions?
Yes! The limiting reactants can change depending on the conditions of the reaction, such as temperature and pressure, which can affect the reactivity and availability of certain reactants. It’s essential to assess the environment when determining limiting factors.
4. What are some common laboratory techniques for determining limiting reactants?
Common techniques include quantitative analysis methods, such as titrations or gravimetric analysis, and working closely with precise measurements to ascertain when one reactant is depleted compared to another during the reaction.
5. How can I apply stoichiometry in cooking?
In cooking, stoichiometry can be employed by adjusting ingredient amounts based on the main component of a recipe—like determining how much salt is needed for a specific volume of water—ensuring a balanced output similar to determining the limiting reactant in chemistry.