Effective Ways to Calculate Percent Yield: A Practical Guide for 2025
Calculating percent yield is a fundamental concept in chemistry that helps scientists and researchers determine the efficiency of their reactions. Percent yield calculation is critical not just in academia, but also in industrial applications, where maximizing efficiency can lead to significant cost savings and resource optimization. This guide will delve into various methods for calculating percent yield, exploring examples, equations, and their significance in chemical processes.
Understanding the Basics of Percent Yield
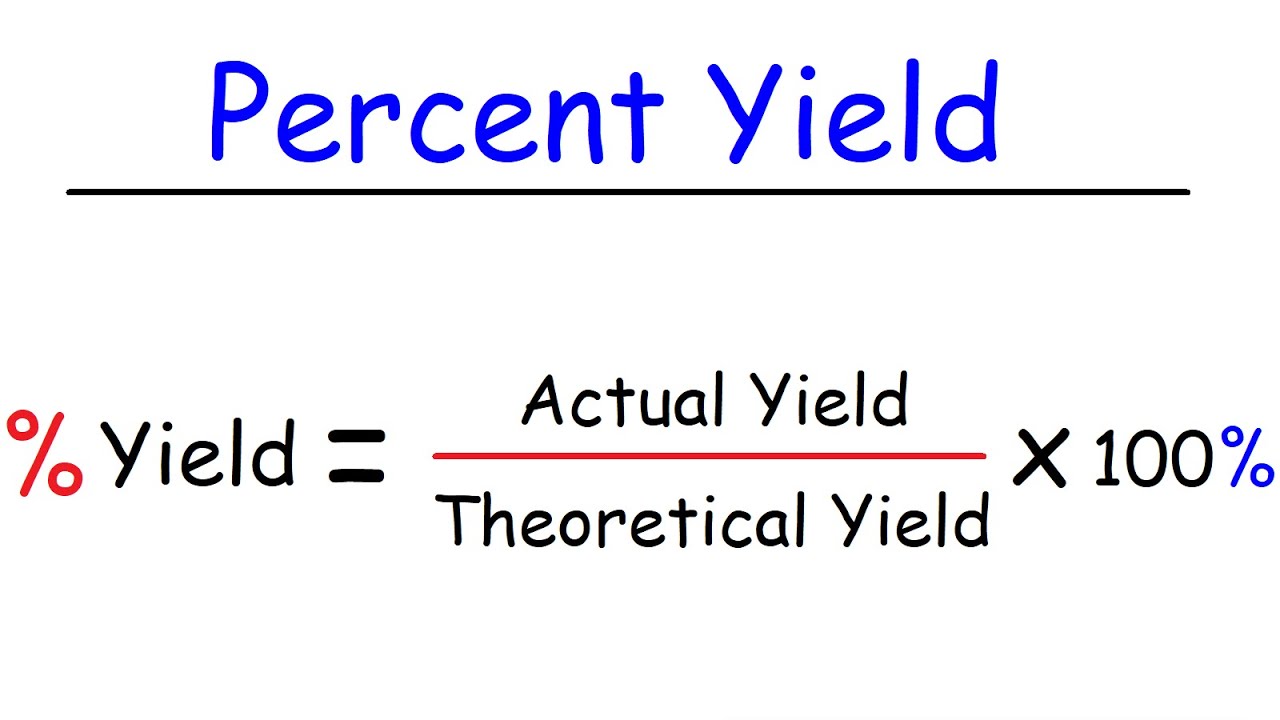
Percent yield is a measure of the efficiency of a reaction, expressed as a percentage. It compares the actual yield of a chemical reaction to the theoretical yield, which is the expected outcome based on stoichiometry. In essence, **calculating percent yield** enables chemists to evaluate the success of their experiments and refine their methods for better outcomes. The basic formula to determine percent yield is:
Percent Yield Formula:
Percent Yield = (Actual Yield / Theoretical Yield) × 100%
Definitions and Key Concepts
To grasp the concept of percent yield properly, it’s crucial to understand the definitions of actual yield and theoretical yield. The **theoretical yield** refers to the maximum amount of product that could be produced from the reactants, assuming complete conversion without losses. On the other hand, the **actual yield** is the amount of product obtained from an experiment. As discrepancies between these two values occur, it’s essential to accurately measure and report each to compute an accurate percent yield.
The Importance of Percent Yield
The **significance of percent yield** lies in its ability to reflect the efficiency of chemical reactions. High percent yields indicate that reactions are operating efficiently, which is critical for cost-effective chemical production. Conversely, low yield percentages may indicate issues such as side reactions, incomplete conversions, or losses during handling. Monitoring percent yield is essential for researchers aiming to improve protocols and enhance productivity.
Methods for Calculating Percent Yield
There are various methods to calculate percent yield in a laboratory setting. Below, we explore the most effective techniques, providing clear examples for better understanding.
Basic Steps in Percent Yield Calculation
**Calculating percent yield** can be done systematically by following these steps:
- Collect your reactants and pure product.
- Determine the theoretical yield using stoichiometry from a balanced equation.
- Measure the actual yield from your chemical reaction.
- Insert the values into the percent yield equation mentioned earlier.
For instance, if you expect to produce 10 grams of product theoretically (theoretical yield), but you only collect 8 grams (actual yield), the percent yield is:
Percent Yield = (8g / 10g) × 100% = 80%.
Using Tables for Yield Calculations
A practical approach to **yield calculations in experiments** can involve using tables to organize data accurately. You can create a table listing every reaction, its expected theoretical yield, actual yield, and calculated percent yield. This method streamlines the process, allowing for easier comparisons while making it simpler to notice trends over multiple experimental runs.
Case Study: Yield Performance Analysis
To further understand the concept of yield, consider the case of synthesizing a desired compound in a laboratory experiment. After thorough experimentation, a chemist calculates theoretical yield based on stoichiometric equations. Observing that their **percent yield performance** varied within different batches gave insights into potential inconsistencies during synthesis phases.
Challenges and Considerations in Percent Yield Calculation
While calculating percent yield may seem straightforward, certain challenges may arise. Understanding these barriers can help researchers implement effective countermeasures to achieve accurate results.
Factors Affecting Yield Percent
Several factors can influence the **importance of yield** including reaction conditions, purity of reactants, reaction time, temperature, and even equipment variations. Identifying and controlling these factors helps ensure a higher **percent yield ratio** which is critical for successful laboratory practices.
Common Mistakes in Yield Calculations
Precision in measurements is at the heart of correct yield calculations. Common mistakes such as inaccurate weighing of products, not accounting for impurities, or incorrectly balancing chemical equations, can lead to misleading percent yields. Hence, it is vital to approach calculations methodically, verifying all data before inputting values into formulas for **chemical yield calculation**.
Raising Your Percent Yield
Improving your **efficient yield calculation** involves optimizing variables within experimental setups. Strategies like maintaining consistent temperature, ensuring complete reaction time, and using high-purity reagents can significantly **raise percent yield**. Monitoring these aspects regularly will enable researchers to develop repeatable methods that also enhance overall productivity in chemical synthesis.
Practical Applications of Yield Calculation
The application of percent yield calculations extends beyond the laboratory into various industries, especially in pharmaceuticals, where maximizing outputs can save costs and time.
Impact on Industrial Chemical Processes
In industrial settings, an understanding of **yield in chemical reactions** facilitates better planning and resource allocation. It prompts manufacturers to evaluate their processes regularly to identify areas of improvement, implementing best practices that can lead to higher production efficiencies while minimizing waste in materials.
Research and Development Insights
In R&D departments, consistently calculating and analyzing yield informs decisions about pursuing certain compounds or methods. If a compound repeatedly yields below desirable levels, researchers may pivot to alternative pathways or adjust reaction parameters to improve overall effectiveness.
Educational Importance in Chemistry
Moreover, the concept of calculating yields plays a crucial role in educational settings, where students learn foundational chemistry. Understanding percent yield equips future chemists with necessary skills for evaluating reaction efficiency, making them more competent when they transition into professional roles.
Key Takeaways
- Percent yield is vital for assessing the efficiency of chemical reactions.
- The percent yield formula is calculated by comparing actual yield to theoretical yield.
- Control factors like reaction conditions to improve percent yields.
- Yield calculations are critical in both industrial processes as well as educational environments.
- Regular assessment of percent yield can lead to more effective and financially sound chemical processes.
FAQ
1. What is the significance of theoretical yield in percent yield calculations?
Understanding the **significance of theoretical yield** is essential; it serves as a benchmark against which actual yield is measured. Theoretical yield helps chemists identify the maximum potential output of a reaction, highlighting the efficiency of execution and guiding necessary adjustments for future runs.
2. How can one maximize yield in chemical reactions?
Maximizing yield often involves fine-tuning reaction conditions, such as temperature and pressure, enhancing reactant purity, and limiting side reactions. By consistently evaluating and adjusting these variables, chemists can achieve higher **yield efficiencies** in their processes.
3. What are common mistakes when performing percent yield calculations?
Common mistakes can include miscalculating the theoretical yield, inaccurate measurement of actual yield, or neglecting impurities during assessments. These errors typically skew results and poorly inform the understanding of reaction efficiencies.
4. Why is understanding actual versus theoretical yield critical?
Grasping the difference between **actual versus theoretical yield** is vital for determining how well a reaction has performed. This understanding allows chemists to pinpoint inefficiencies, optimize procedures, and ultimately advance their expertise in chemical synthesis.
5. Can yield calculations influence decisions in research and development?
Indeed, **yield calculations** play a critical role in R&D as they provide insights needed to shift focus from less efficient methods or compounds. This information allows scientists to strategize effectively, maximizing resources, and optimizing outcomes in chemical experimentation.